By Dr. Avijit Das
In the microscopic world of infection, survival often depends on unlikely alliances. In the case of Listeria monocytogenes, a dangerous foodborne pathogen, that ally is a virus quietly embedded in its genome — a virus that doesn’t kill, but helps it hide and thrive inside our bodies.
When we think of viruses infecting bacteria — the infamous bacteriophages — we often imagine a destructive invasion: the virus hijacks its host, multiplies rapidly, and ultimately bursts the bacterium open in a violent release. But in nature, some phages take a quieter path, embedding themselves into the bacterial genome as dormant passengers, or prophages. And sometimes, they do more than just sit around.
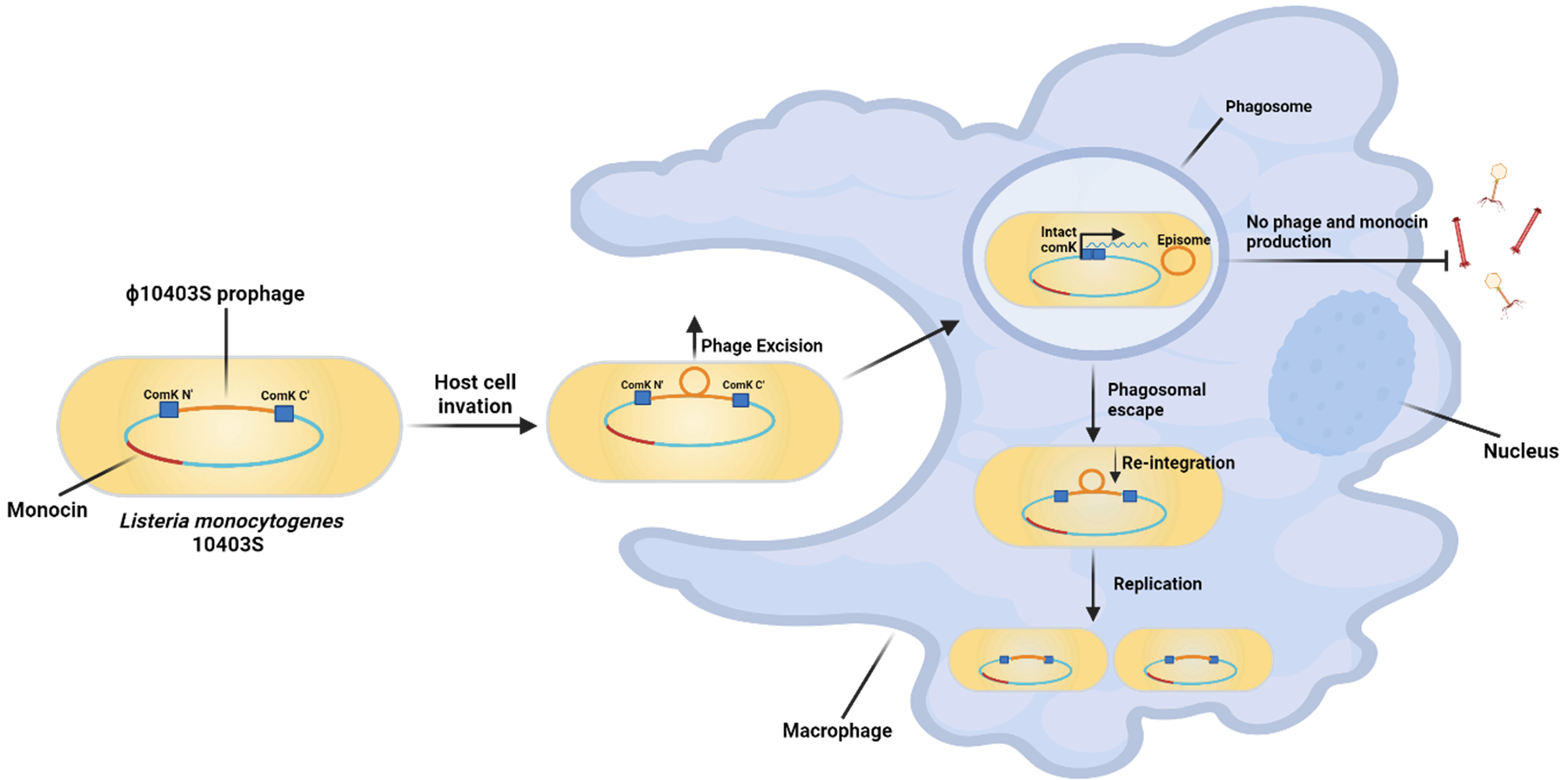
In a fascinating twist, scientists in the Herskovits lab at Tel Aviv University have discovered that one such prophage plays an active, almost cooperative role in the infection process of Listeria monocytogenes (Lm). This bacterium, notorious for causing listeriosis — a serious infection especially dangerous to pregnant women and immunocompromised individuals — carries a phage integrated within a gene called comK. But here’s the kicker: when Lm infects macrophages, the body’s frontline immune cells, the phage excises itself from comK, restoring the gene’s function just in time to help the bacterium escape the immune cell’s digestive chamber, the phagosome.
This strategic excision enables Lm to burst out of the phagosome and into the host cell’s cytosol, where it can continue its infectious journey. But while this maneuver sounds like the start of a classic viral takeover, what happens next is highly unusual.
An Unusual Kind of Alliance
Unlike typical lytic phages, which proceed to produce viral particles and eventually kill their bacterial host, this prophage stops short of full activation. The early phage genes are turned on inside the host cell — but the late-stage genes, responsible for producing new virions and causing bacterial lysis, remain completely silent. No viral particles, no destruction. The bacterium survives. The phage, embedded in its host, also persists.
This behavior has been dubbed “active lysogeny”, a term describing prophages that take an active role in helping their bacterial host — not destroying it. It represents a new understanding of how phages can shape bacterial pathogenicity without initiating the full viral cycle.
The Temperature-Sensitive Switch
So how does this phage know when to stop? The answer may lie in temperature — and a remarkable bit of molecular regulation.
The late-stage phage genes are known to be activated by a phage-encoded transcription factor called LlgA, part of a family of proteins involved in initiating the lytic phase. However, research suggests that LlgA doesn’t get to act freely inside a mammalian host. Instead, its levels are tightly controlled — and the key to that control seems to be temperature.
At the cooler temperatures found outside the body, LlgA functions normally. But at the body’s internal warmth, something changes: the protein appears to be degraded or modified, likely through a process akin to regulated proteolysis. Early findings suggest that the bacterium itself mediates this regulation, using specific proteases to cleave LlgA and prevent the activation of lytic genes — a clever mechanism to preserve the bacteria–phage alliance without risking host suicide.
Toward Smarter Phage Therapies
The implications of this research go far beyond understanding Listeria. As antibiotic resistance grows and phage therapy re-emerges as a potential weapon against drug-resistant bacteria, knowing how phages can cooperate with — or be controlled by — their hosts becomes increasingly important.
If scientists can decipher how LlgA is regulated, identify the proteases involved, and understand the structure of the active and inactive forms of this protein, we may unlock new strategies for designing therapeutic phages. Instead of relying on phages that indiscriminately kill bacteria, future therapies could use genetically engineered phages that are active only under specific conditions — or cooperate with host bacteria in beneficial ways.
The research underway aims to map this regulation in molecular detail, providing insights into not only Lm pathogenesis but also the broader, often overlooked world of temperate phages as strategic partners in microbial life.
In the war between bacteria and the immune system, sometimes the most powerful weapons are the ones you never see coming.